Your cart is currently empty!
FDA 510(k) Clearance Regulatory Consultation for Medical Devices and IVDs
Why Companies Choose NAMSA
FDA Pre-Submission Meetings Managed Each Year
Medical Device and IVD Manufacturers Supported Each Year
Consultants with Previous US FDA or EU Notified Body Experience
Getting You From Application to Approval as Quickly as Possible
The US accounts for more than 40% of the global medical device market. While many device manufacturers are intimidated by the process of gaining 510(k) clearance for their medical device, the FDA provides a wealth of information on the requirements, and is generally accessible and helpful. Still, the sheer abundance of relevant FDA guidance documents can be overwhelming and requirements often get missed or misinterpreted. NAMSA’s regulatory team has deep experience preparing the data needed to support your 510(k) application and can assist with every step along the way.
Our Regulatory Consultants Understand What Data FDA Reviewers Expect to See
As one of the world’s leading medical device and IVD testing and clinical research organizations, we fully understand the importance of the data that forms the backbone of your 510(k) submission. We have supported FDA and EU regulatory submissions for thousands of medical devices and In Vitro Diagnostic devices. Numerous members of our team have previous FDA or Notified Body experience, giving them a unique insight into how the process works and the pitfalls to avoid. Our team has deep experience in a wide variety of device categories including:
- Cardiovascular
- Dental
- Diagnostic Imaging
- In Vitro Diagnostics
- Neurology
- Oncology
- Ophthalmology
- Orthopedics
- And many more
Five Ways NAMSA Can Assist With Your 510(k) Submission
- Strategy and Regulatory Pathway – Manufacturers that try to prepare a 510(k) submission themselves often veer down an erroneous path, completing unnecessary testing or the wrong testing altogether. It is also very common for companies to select the wrong product code. Our team will lay out the precise pathway needed to secure 510(k) clearance along with all required testing, data and other requirements, including quality system management procedures.
- Gap Analysis – Companies usually come to us with some basic testing data already collected on their device. Our consultants can conduct a comprehensive gap analysis to determine where shortcomings exist and how to fill them. Again because we have so much background in testing and clinical data our teams can work collaboratively to make sure that you meet all FDA 510(k) clearance requirements for your specific product code. We will also carefully review your chosen predicate device to ensure it is appropriate.
- FDA Pre Submission Meetings – The FDA offers a program that allows manufacturers to meet with reviewers well before a 510(k) submission. This is extremely helpful and advisable if there’s any question about applicable guidance for your device or the data that will be required. This is sometimes the case when a product is more complicated or innovative and FDA guidance is not clear. We can help you prepare for those meetings and lead them on your behalf.
- Preparation of your 510(k) Submission –The success of your FDA submission ultimately depends on the quality of the data that you provide, but our regulatory team is skilled at reviewing what you have and addressing issues early on in the process. We will then carefully compile your data following the FDA’s required format, minimizing the chances that you’ll receive a Refuse to Accept (RTA), Additional Information (AI) request, or worse yet, a Not Substantially Equivalent (NSE) determination.
- Post 510(k) Submission Support – While our regulatory team does everything possible to follow FDA regulations and guidance documents for your specific product code, FDA reviewers may still have questions about the data submitted. In this case, FDA will send a letter requesting Additional Information. We will help you navigate that delicate process and follow up with the FDA in a manner that satisfies their need for more information, quickly and cost effectively.
Market Expansion
If the US is your first market for this device, your future plans may involve expansion to Europe, Canada, Japan or other markets. Because we have experience with all of these markets we will guide you on which standards to follow and how to approach data collection so you have all the data needed to smooth your expansion into these markets. We can also advise when the same data can be used for multiple regions and when you may need additional or separate data to support a given region.
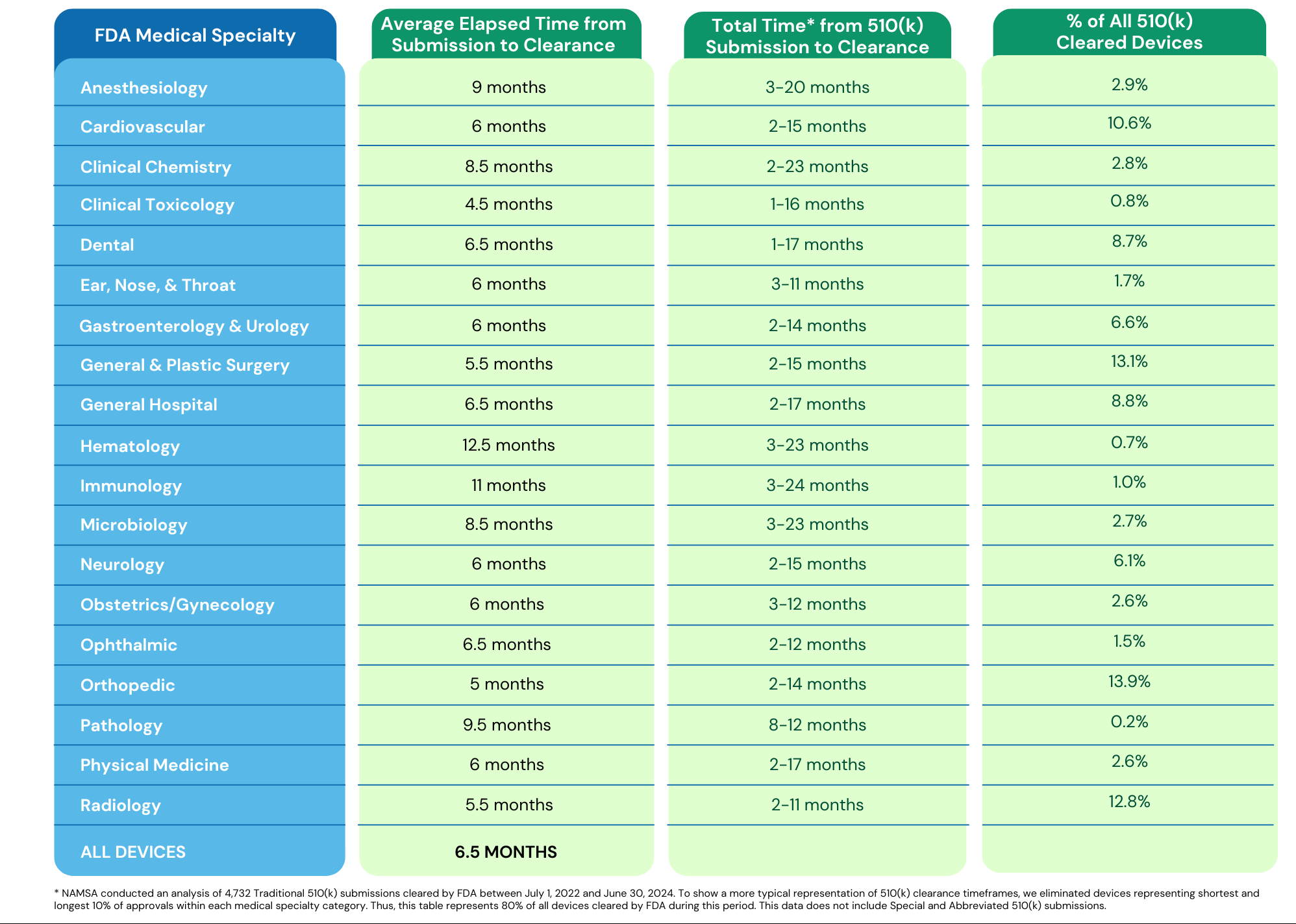
How Long It Takes to Get 510(k) Clearance
The FDA has a goal to review 95% of all their applications within 90 days. The reality is that most submissions result in an Additional Information request which stops the clock with FDA. Nonetheless, companies that respond to these requests quickly are able to gain 510(k) clearance within six to nine months.
Get to Market Faster with NAMSA’s APEX PROGRAM
If getting your product to market as fast as possible is essential to the profitability of your company, consider our APEX PROGRAM. The APEX PROGRAM is our service which pairs you with a single senior NAMSA consultant who will guide and speed you through all aspects of data collection, 510(k) preparation, and ultimately, regulatory submission.
Meet Our FDA Consultants
Meet the team ready to put their expertise to work for you.
-
Carla M. Wiese, BS-Mech Eng
 Principal Strategy Consultant, RegulatoryView Bio
Principal Strategy Consultant, RegulatoryView Bio -
Sarah B. Nelson, MS
 Senior Strategy Consultant, RegulatoryView Bio
Senior Strategy Consultant, RegulatoryView Bio -
Monica R. Montañez, MS, RAC, CQA
 Principal Strategy Consultant, RegulatoryView Bio
Principal Strategy Consultant, RegulatoryView Bio -
Adam Saltman, PhD, MD
 Principal Strategy Consultant, Clinical and Regulatory ServicesView Bio
Principal Strategy Consultant, Clinical and Regulatory ServicesView Bio -
Richard A. Vincins, BS, CMDA, CQA, RAC-Devices, M-TOPRA, CQP MCQI
 Principal Strategy Consultant, RegulatoryView Bio
Principal Strategy Consultant, RegulatoryView Bio
Other FDA Services That May Interest You

FDA De Novo Submissions

FDA PMA Consulting
