The following is an excerpt from ENDOVASCULAR TODAY, VOL. 20, NO. 10, 93 (OCTOBER 2021); to access the full article, please click here.
The field is at a crossroads where opportunities must be seized to establish a permanent foothold in the crowded world of oncology care.
The flight home from this year’s American Association of Clinical Oncology (ASCO) meeting was particularly satisfying. Not only was the flight especially fast, but more importantly, any lingering doubts about interventional oncology being a mainstay within the crowded multidisciplinary oncology care have vanished. “We finally made it!” gleefully exclaimed an old pioneer of this field sitting next to me on the plane, who, like me, is past retirement age and ready to savor this precious moment. For many years, we had fought to establish the field of interventional oncology as a legitimate fourth pillar of oncology, and then just as we were about to succeed, the immuno-oncology revolution arrived, and with it the acronym “IO” was no longer synonymous with interventional oncology but rather immuno-oncology! But this ASCO meeting—filled with oral presentations and lectures about the benefit of combining locoregional with systemic therapies, scientifically robust clinical trial data from new cutting-edge technologies solidly anchored in interventional oncology, and the exponential growth of biomedical imaging, including molecular imaging and artificial intelligence (AI)—has cemented the role of the real IO (meaning interventional oncology) in the minds of our oncology colleagues, industry leaders, and the public at large.
Is this wishful thinking, a futuristic illusion, or truly the path that lies ahead for interventional oncology? Let’s come back to the present in 2021. What will it take for the youngest and most rapidly growing offshoot of interventional radiology to gain that permanent foothold within oncology?
The Need for Scientifically Sound Clinical Data
As cancer has surpassed cardiovascular diseases and is now the leading cause of death worldwide, its conquest remains elusive despite recent notable progress. Cancer remains an incredibly complex disease, involving virtually every tissue in the body and affecting many genes. The investment in cancer research continues to be enormous, and progress has clearly been made in cancer cell genetics, biochemistry, and function, but a cure is still far away. This presents an opportunity for interventional oncologists because the need for local control of cancer remains an important aspect of the overall therapeutic strategy. As a result, a meaningful collaboration between various specialties involved in cancer care—including interventional oncology—has been highlighted as a critical need. The new era of cancer research is here, and interventional oncology can play its part to bring about therapeutic benefits for cancer patients. However, in order to “belong,” interventional oncology must evolve away from single-institution, retrospective, underpowered reports that have no influence on clinical practice to meaningful prospective multicenter clinical trials that offer significant outcomes.
To that end, the recent data on yttrium-90 (Y-90) radioembolization for liver cancer are compelling because they show that the field of interventional oncology is indeed capable of generating the much-needed (and long overdue) data to lead to adoption of therapies anchored in interventional oncology, such as radioembolization. During this past year, several studies have shown the importance of both the absorbed dose by the tumor and the relationship between baseline imaging, preprocedure technetium 99m (99mTc)- macroaggregated human albumin (MAA) single-photon emission CT (SPECT)/CT, and immediate post– Y-90 radioembolization SPECT/CT. A secondary analysis of the SARAH study demonstrated the close association between tumor radiation–absorbed dose and improve ment in overall survival and disease control at a threshold of 100 Gy, whereby patients who received ≥ 100 Gy had a much higher median overall survival than those who received < 100 Gy (14.1 vs 6.1 months; hazard ratio [HR], 0.38; P < .001).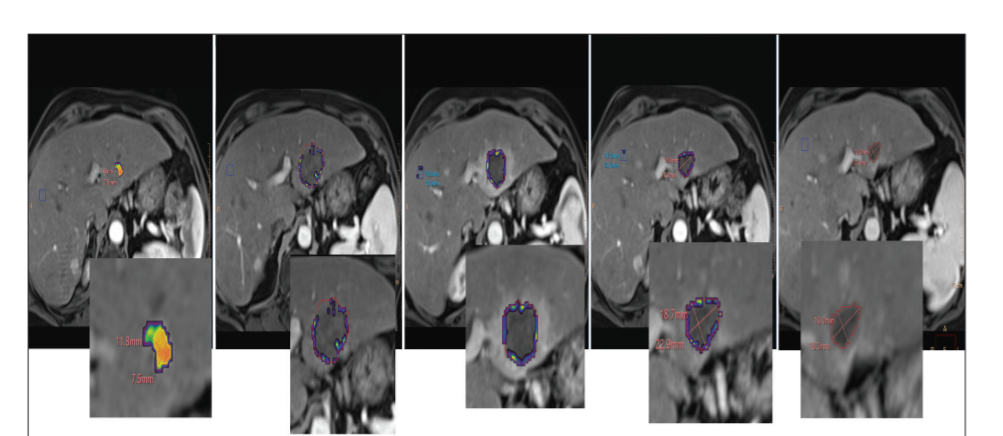
Figure 1. MRIs obtained at baseline and 24 hours, 1 week, 1 month, and 2 months posttreatment (from left to right) with histotripsy in a patient with a small hepatocellular carcinoma in segment 2/3. The three-dimensional measurement known as qEASL (quantifiable European Association for the Study of Liver) was performed using a semiautomatic tumor segmentation software (Philips Healthcare). The qEASL color map is overlaid on the subtracted MRI, showing the enhancing portions of the tumor in red/yellow before treatment and complete lack of enhancement after treatment consistent with a complete response to histotripsy (bottom row images are magnified views of the MRIs in the top row).
More remarkably, when the visual agreement between baseline CT imaging, pre-procedure 99mTc-MAA SPECT/CT, and immediate post– Y-90 radioembolization SPECT/CT was optimized and coupled with an absorbed tumor dose of ≥ 100 Gy, the results were even more impressive (24.9 vs 6.7 months; HR, 0.24; P < .001).3,5 The DOSISPHERE study demonstrated that the implementation of a personalized dosimetry approach including a tumor-absorbed dose > 205 Gy resulted in significantly better outcomes (overall median survival, 26.6 vs 10.7 months; HR, 0.421; P = .0096). These two studies clearly show that the concept of radioembolization as we knew it has been radically changed for the better. Thus, careful treatment planning in the form of dosimetry planning is now indispensable to maximize the tumor-absorbed dose and achieve the best outcomes both in terms of tumor response and patient survival.
To access the full article, please click here.
How Can NAMSA Help?
The global medical device landscape is complex. Getting a new product or therapy approved requires compelling clinical evidence. At NAMSA, we know what you’re up against and understand how to set you up for success.
Our clinical expertise spans every manner of technology, therapy, indication and geography. This broad range of experience allows us to successfully lead our clients through all phases of clinical research: from first-in-human to pivotal and post-market. NAMSA’s global footprint also provides clients direct access to our local networks to conduct safe, effective and efficient clinical trials, which are optimized to achieve regulatory approval and continued innovation.
To learn about NAMSA’s full suite of Clinical Research Services, including Interventional Oncology, please visit: https://namsa.com/services/clinical-research/.
Dr. Jean-Francois H. Geschwind
Jean-Francois (Jeff) H. Geschwind, MD is currently the Medical Director for Oncology and Image-Guided Therapy at NAMSA, Director of Oncology at USA Clinics Group and a Consultant for Cage Pharma, previously PreScience Labs, a company he founded in 2009 which is focused on developing cancer drugs targeting tumor metabolism. He is also a Scientific Consultant for Philips Healthcare, advising them on all matters related to oncology and image-guided therapy. Dr. Geschwind is internationally recognized as an expert and key opinion leader in liver cancer. He was the lead or co-investigator on more than 50 clinical trials testing innovative treatments for patients with liver cancer funded by pharmaceutical companies, foundations and/or the NIH. He also received multiple patents for research on a new promising drug that targets a specific pathway in cancer cells, which allowed him to create a company based on this discovery. Dr. Geschwind has been recognized as a Top Doctor by US News and World Report, as well as a Top Radiologist by Top Doctor Magazine. He has also been a Diplomat of the American Board of Radiology since 1998.
