On 4 October 2021, the Medical Device Coordination Group (MDCG) released the long-awaited guidance document (MDCG 2021-24) on classification of medical devices under MDR 2017/745. The MDCG document utilizes the same format as MEDDEV 2.4/1 Rev 9 as it relates to the classification of medical devices under MDD 93/42/EEC. Therefore, direct comparisons can be made to indicate changes in classification requirements between the MDD and MDR.
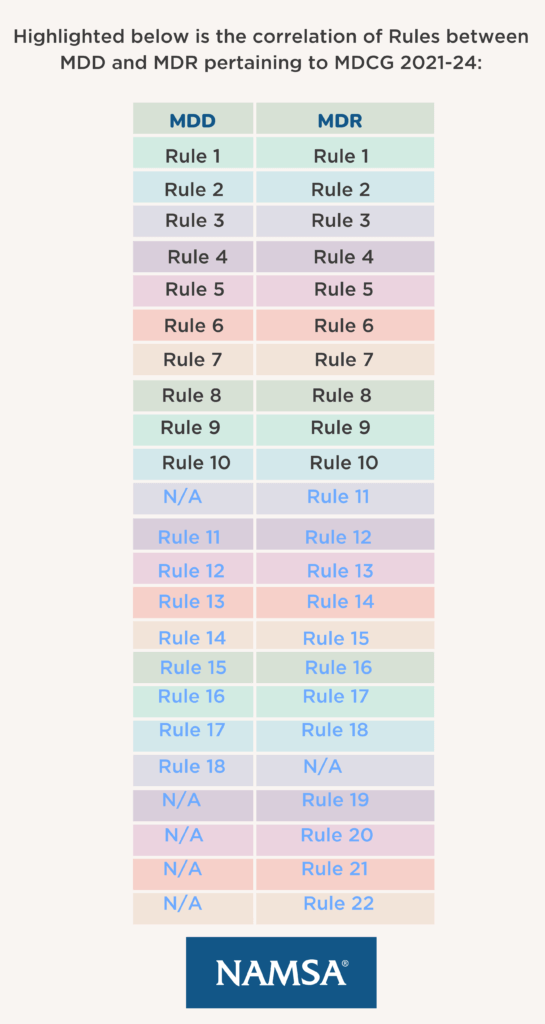
The MDCG document, helpfully, still includes sections on definitions (section 3), flow diagrams for each rule (section 4.1) and examples of devices covered by each rule (section 4.2). However, the rule numbers do not correlate directly between MDD and MDR due to the new rule for Software (Rule 11) and four additional special MDR rules.
NOTEWORTHY CHANGES
Following is a breakdown of the most significant changes in the new MDCG document compared to the previous MEDDEV.
I. DEFINITIONS
- A definition for “Intended Purpose” is no longer included in the MDCG document; instead a definition of “Specific Medical Purposes” has been included.
- The definition of “Continuous Use” has been modified and expanded to give greater clarification.
- A new definition of “Injured Skin or Mucous Membrane” has been added.
- The definition of “Active Devices” has been modified to remove specific reference to “Electrical Energy” and refers only to ‘Energy.’
- Software and not just “Stand Alone” software is defined as an ‘Active Device;’ also, software must be reviewed not just in relation to Rule 11.
- Specific definitions of “Active Therapeutic” and “Active Diagnostic and Monitoring” devices are now included.
- The definition of “Devices with a Measuring Function” has been significantly expanded, including criteria which must be met by the device.
- The definition of a procedure pack now includes a combination of medical devices, IVDs and other products which comply with their relevant legislation.
- The following terms have been added and defined
- “Systemic Absorption”
- “Wholly or Mainly Absorbed”
- “Local Dispersion”
- “Medicine/Medicinal Product”
- “Nanomaterial” and “Derivative”
II. CLASSIFICIATION ON DISPUTE PROCESS
The process for handling classifications disputes has been changed. The lead Competent Authority which adjudicates on the dispute is changed from the Competent Authority which oversees the Notified Body to the Competent Authority where the manufacturer or EU Authorized Representative has their registered place of business. When the Notified Body is located in a different member state, then the Lead Competent Authority must consult with the Competent Authority which oversees the Notified Body before a final decision is made.
III. MAIN CHANGES TO RULES (Rule Numbers Relate to MDR)
A. Non-Active Device Rules:
- Rule 2: Blood Bags (Class IIb) have been added. Note: Blood bags incorporating heparin or other substances such as anticoagulant agents (if used separately) can be considered a medicinal product as covered by Rule 14; these are considered Class III devices.
- Rule 3: Devices consisting of a substance or a mixture of substances intended to be used in vitro in direct contact with human cells, tissues or organs taken from the human body or used in vitro with human embryos before their implantation or administration into the body (Class III) has been added.
- Rule 4:
- Where “Injured Skin” is referenced, Mucous Membrane has been added e.g. “in contact with injured skin or mucous membrane”
- Devices intended for wounds which breach the dermis and heal only by secondary intent (Class IIb) have been added as an option
- Rule 5: Note that Rules 4, 20 and 21 may also be relevant
- Rule 6: Note that Rule 4 may also be relevant
- Rule 8:
- Active Implantable Devices or their Accessories (Class III) have been added
- Surgical Meshes (Class III) have been added (up-classification from MDD)
- Partial Joint Replacements (Class III) have been added (up-classification from MDD), except components such as screws, wedges, plates and instruments (Class IIb)
- Spinal disc replacement or implants in contact with spinal column (Class III) have been added (up-classification from MDD), except components such as screws, wedges, plates and instruments (Class IIb)
B. Active Device Rules
- Rule 9:
- Note that Rule 22 may be relevant
- Devices intended for controlling, monitoring or directly influencing the performance of active implantable devices (Class III) have been added as an option
- Devices intended to emit ionizing radiation for therapeutic purposes and devices which control or monitor such devices, or which directly influence their performance (Class IIb), have been added as an option
- Rule 10:
- Devices intended to illuminate the patient’s body in the visible spectrum (Class I) have been added; these devices were previously covered by Rule 13 “All Other Active Devices” (Rule 12 under the MDD)
- Devices intended for diagnosis in clinical situations where the patient is in immediate danger (Class IIb) have been added as an option
- Rule 11 is a new Rule specifically relating to Software; depending on the use of the software the classification can range from Class I to Class III
C. Special Rules
- Rule 14: There is a change of wording in the definition:
- MDD = Medicinal substance liable to act in an ancillary way on the human body
- MDR = Medicinal substance that has an action ancillary to that of the devices
- The consequence of this change will be to ensure that all devices with a medicinal aspect will now be covered by this rule (e.g. see blood bags).
- Rule 16: This rule has now added “devices” to become “devices intended specifically to be used for disinfecting or sterilising medical devices,” therefore now fully covers sterilization equipment.
The following Rules are all new to the MDR:
- Rule 19: Devices incorporating or consisting of nanomaterials (Class IIa to Class III)
- Rule 20: Devices invasive in respect to body orifices (not surgical) to administer medicinal products by inhalation (Class IIa to Class IIb)
- Rule 21: Devices composed of substances or of combinations of substances to be introduced into the human body via a body orifice or applied to the skin and absorbed by or locally dispersed in the human body (Class IIa to Class III)
- Rule 22: Active therapeutic devices with an integrated or incorporated diagnostic function which significantly determines the patient management by the device, such as closed loop systems or automated external defibrillators (Class III)
How Can NAMSA Help?
Unsure of the potential impact of MDCG 2021-24 on your organization’s medical device products? NAMSA is the industry leader in driving successful regulatory outcomes through effective interactions with the EU Commission and Notified Bodies. Our internal teams of medical device and IVD development experts communicate with EU entities nearly every day and are the most experienced in industry at accelerating regulatory submissions and approvals for manufacturers. In fact, many of our Associates have previously held positions within these organizations, which provides Clients the benefit of a clearer understanding on how to proactively plan for international requirements and expectations.
To learn about NAMSA’s full suite of Regulatory and Quality services and solutions, including MDR compliance planning, please visit: https://namsa.com/services/regulatory-and-quality-consulting/.
We also invite you to access our complimentary MDR and IVDR Planning Resources, here.
Kevin Butcher
Kevin Butcher is an experienced Senior Manager with extensive knowledge of medical device regulatory requirements, including Product Technical Files and QMS 3rd Party auditing. Mr. Butcher possesses 18 years of Notified Body experience, latterly as Certification Manager for SGS United Kingdom. Kevin joined the medical device consulting team at NAMSA in January 2021 and currently serves as a Principal Regulatory Consultant.
