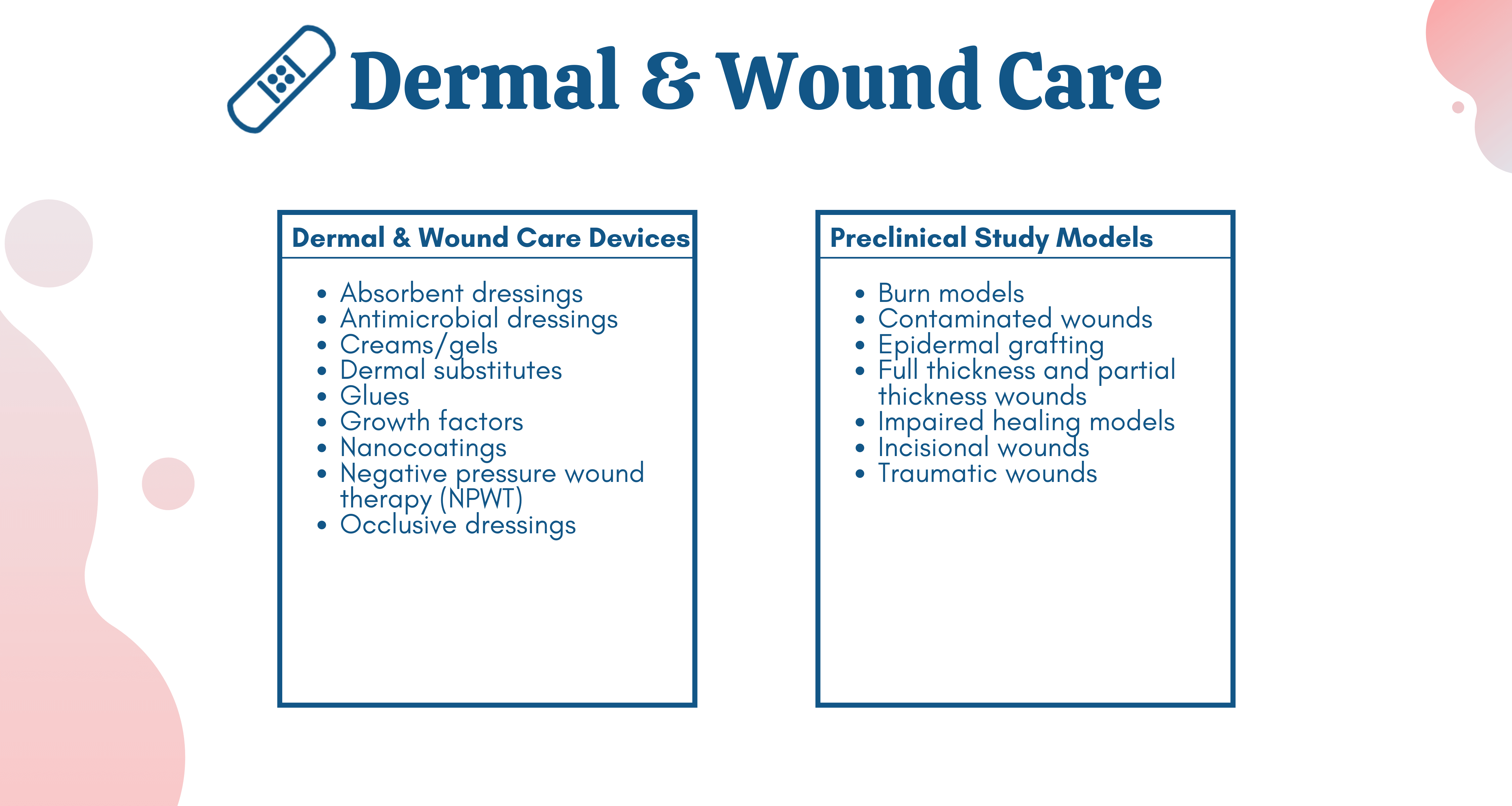
NAMSA has assisted with the successful research, development and commercialization of a wide range of wound healing devices including closures, tissue engineered skin substitutes, traditional and advanced wound management, negative pressure wound therapy (NPWT), ostomy drainage bags, pressure relief, compression therapy, wound debridement, surgical sutures, cold plasma therapies, debridement products, irrigation solutions, dressing removal agents, Non-Medicated Wound Dressings (NMWD), complex adjunct therapies and beyond.
Our team of experts can help make sure that the wound care products you develop meet the latest regulatory standards — including the European Committee for Standardization’s revised standard EN 13726 test method.
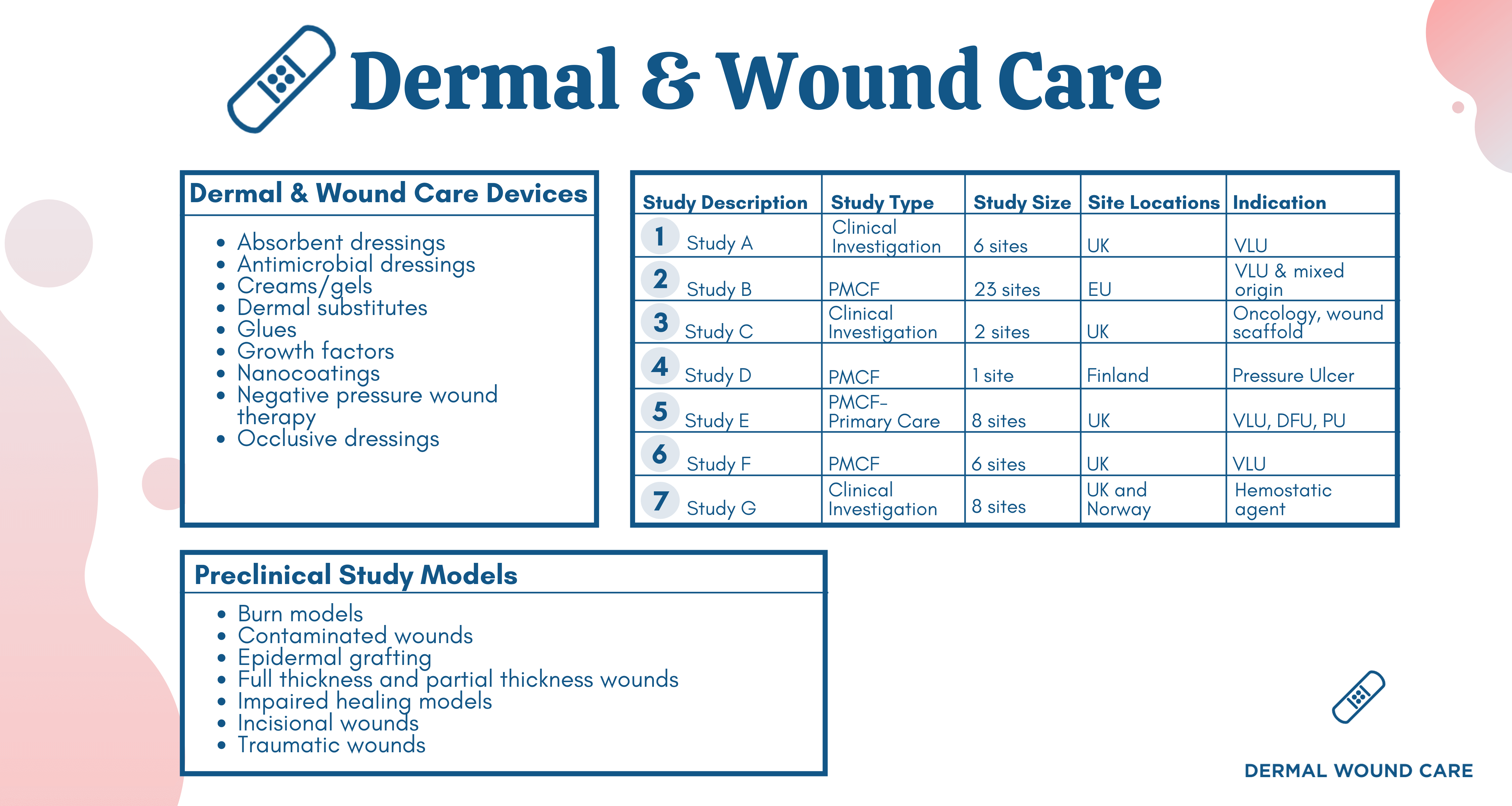
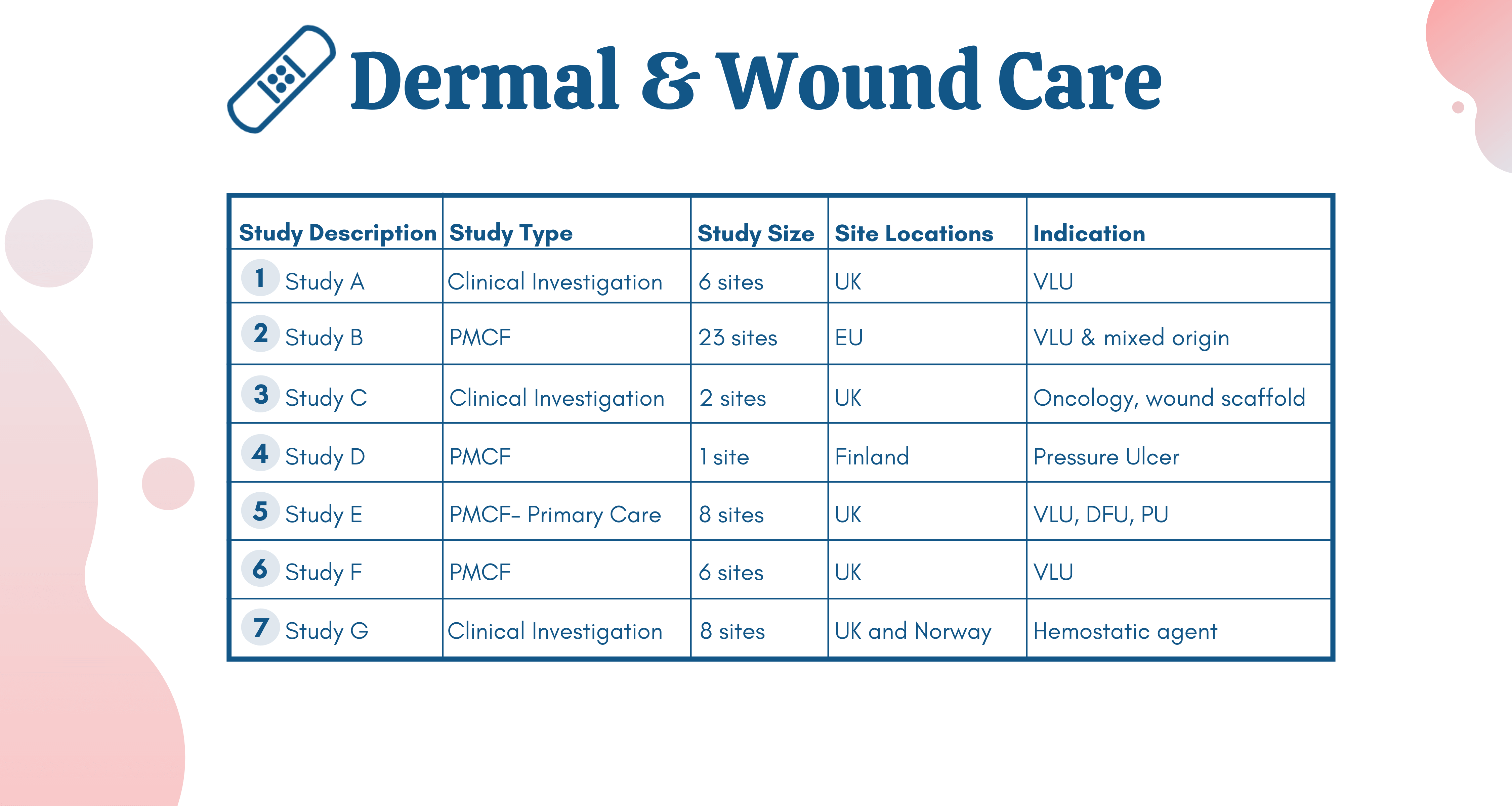
In recent years, NAMSA has completed over 300 wound healing research studies with a range of devices and study models, evidenced below.