Your cart is currently empty!
NAMSA Experience with Peripheral Vascular Devices
Clinical Projects in Support of Cardiology and Vascular Devices
Preclinical Projects in Support of Cardiovascular Devices
Fully Equipped ORs and Cath Labs in US and Europe
Board-Certified DaBT Toxicologists on Staff
Find Help For Your Peripheral Vascular Device
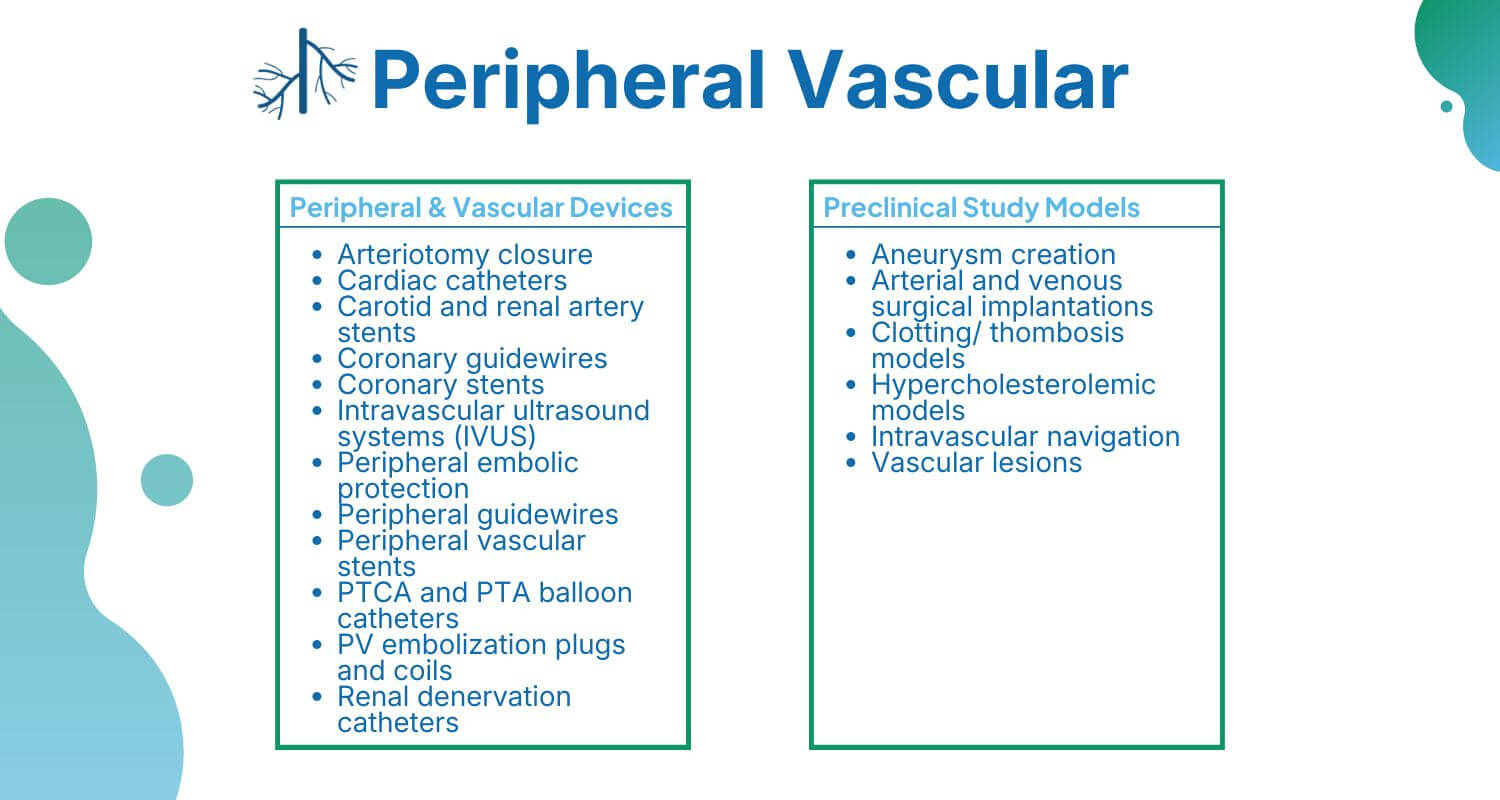
NAMSA is the right match for manufacturers seeking a product development partner with decades of experience helping bring peripheral vascular devices to market. We’ve worked on vascular devices relating to arteriotomy closure; carotid and renal artery stents; peripheral embolic protection; peripheral guidewires; peripheral vascular stents; PTA balloon catheters; PV embolization plugs and coils; renal denervation catheters and more.
Our technical specialists and scientists are adept at developing preclinical testing programs that meet your product’s unique needs. Whether you need help optimizing biocompatibility and functionality of your vascular device or need someone to guide you through global regulatory and reimbursement challenges — our product development specialists will support you every step of the way.
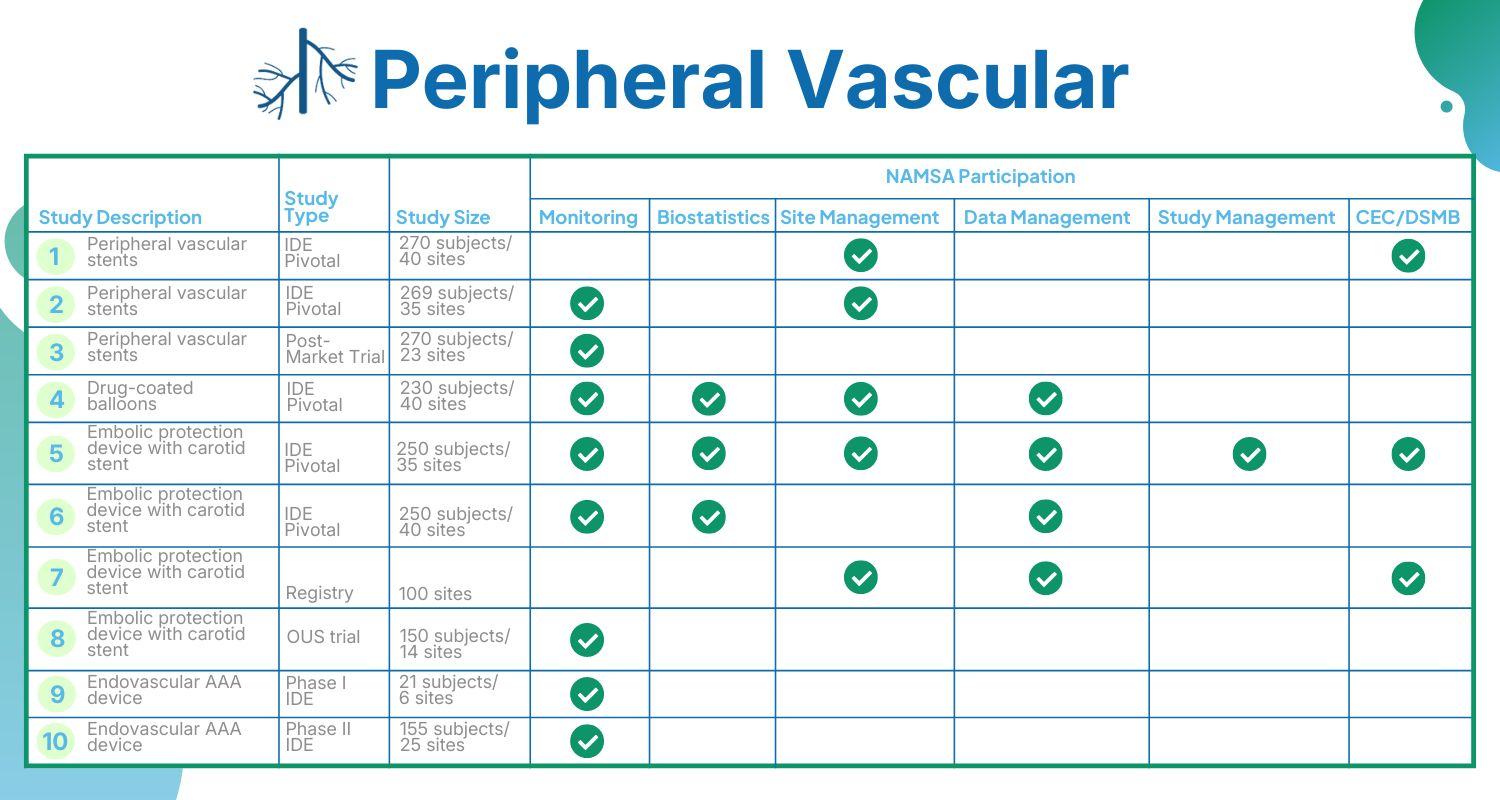
Peripheral vascular device and product development makes up a large portion of the commercialization work we do at NAMSA. We have supported hundreds of cardiology and vascular device clinical trials and customized preclinical studies.