Your cart is currently empty!
NAMSA Experience with with Wound Care Technology
Overview
NAMSA has assisted with the successful research, development, and commercialization of a wide range of wound healing devices including closures, tissue-engineered skin substitutes, traditional and advanced wound management, negative pressure wound therapy (NPWT), ostomy drainage bags, pressure relief, compression therapy, wound debridement, surgical sutures, cold plasma therapies, debridement products, irrigation solutions, dressing removal agents, Non-Medicated Wound Dressings (NMWD), complex adjunct therapies and beyond.
Our team of experts can help make sure that the wound care products you develop meet the latest regulatory standards — including the European Committee for Standardization’s revised standard EN 13726 test method.
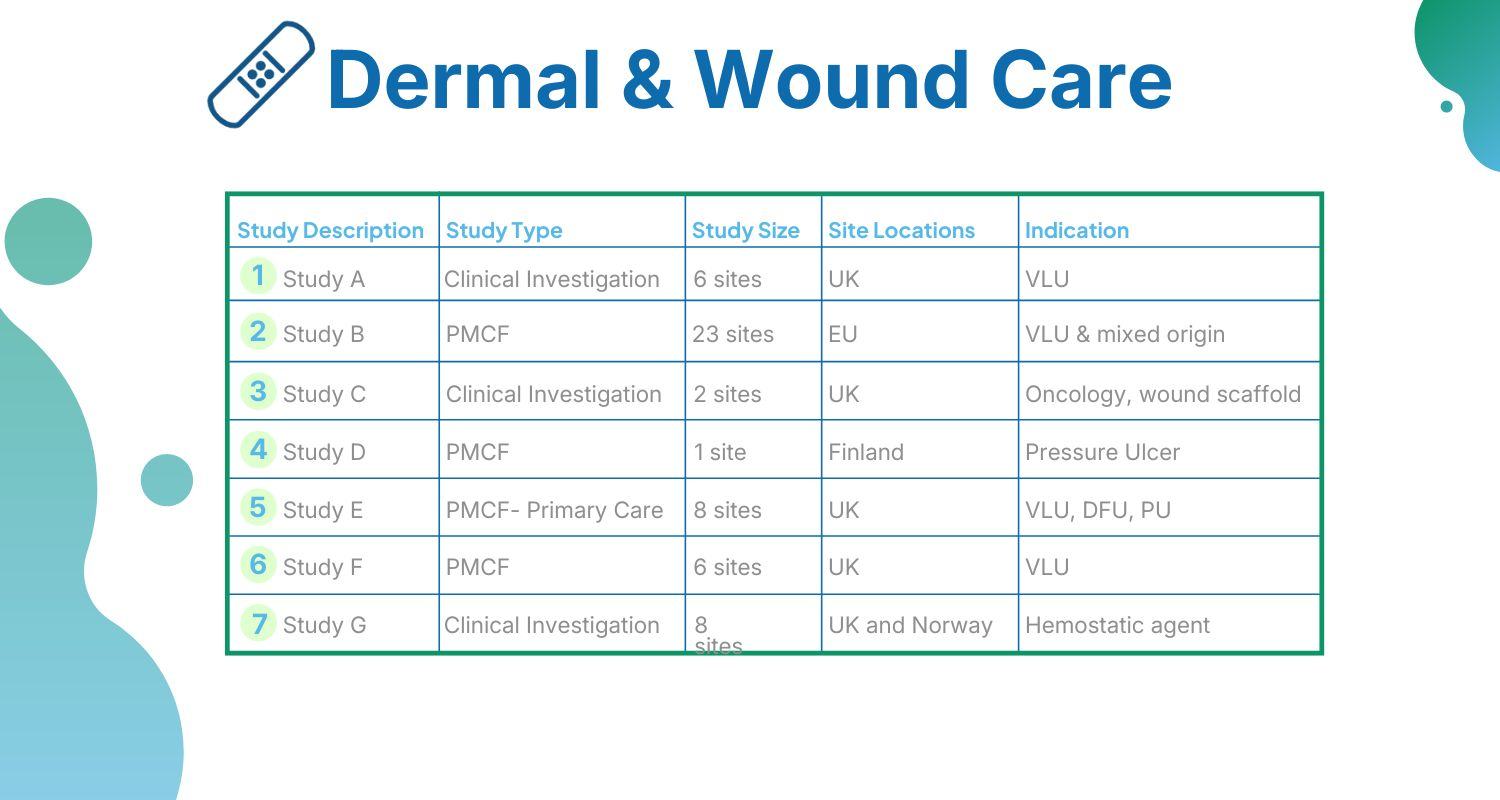
In recent years, NAMSA has completed hundreds of wound healing research studies with a range of devices and study models, as evidenced below.
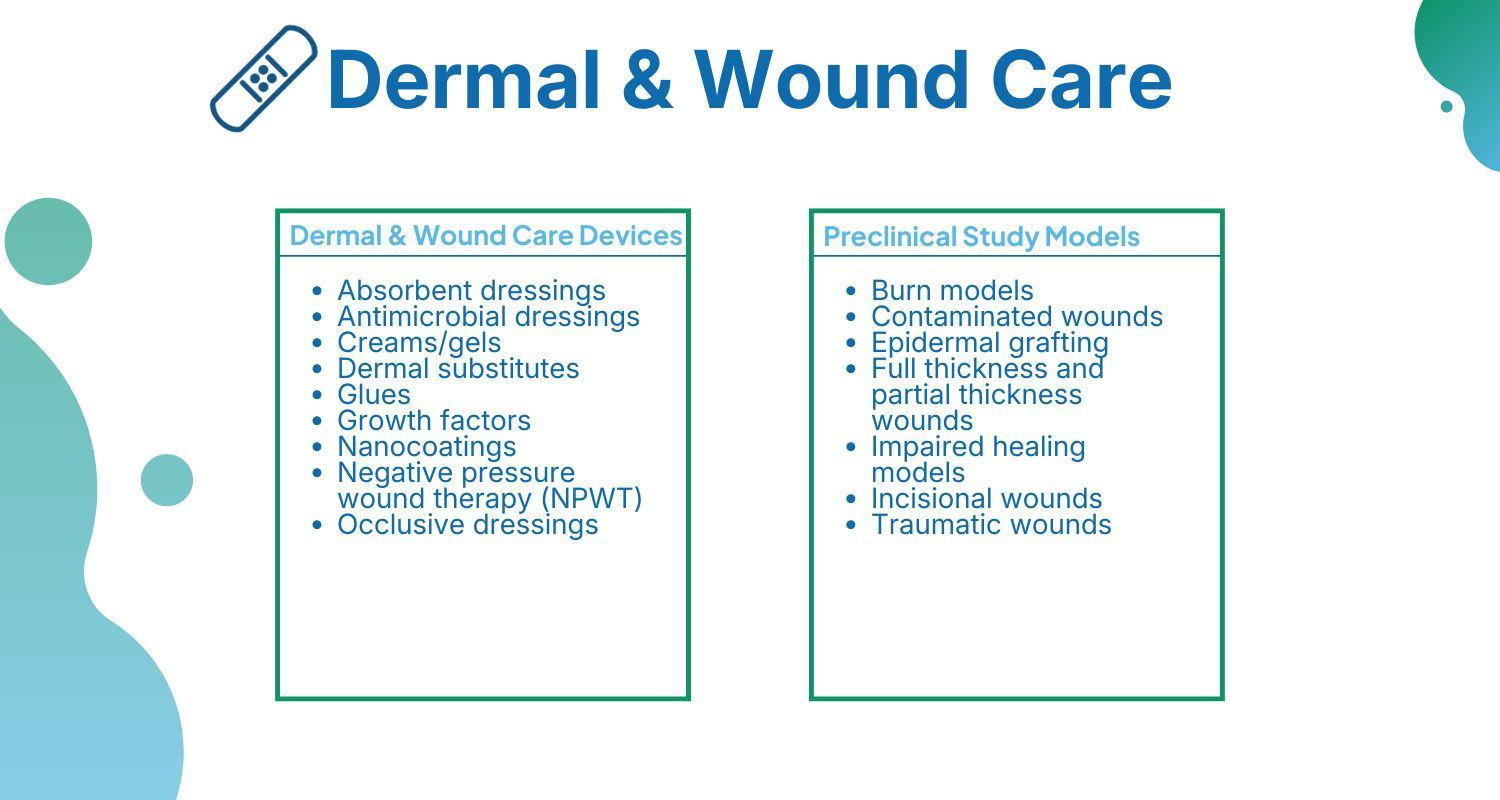
Wound Care Research
Our expert teams can support your wound care research through a continuum of developmental support. This includes preclinical, biocomp, microbiology, materials testing, clinical research studies and global regulatory support as well as:
- Regulatory support for hernia repair/wound healing devices
- Regulatory submission support of several surgical adhesives and resorbable sutures
- Full clinical trial management for Class III surgical adhesives (PMA approvals)
- Pivotal IDE studies for tissue glues
- Full clinical trial management for Class III hemostatic agents
- Study management for abdominal repair meshes
- Microbiology Bioburden testing to determine a quantitative estimation of the number of viable microorganisms associated with medical products, raw materials or tissue samples
- Microbiology Antimicrobial Testing including the AATCC Test Method 100 for antimicrobial finishes on fabrics for Fungus and the ASTM 32149 for determining the antimicrobial activity of immobilized agents under dynamic contact conditions
- Medical device microbiology testing including ISO 17025 accredited biofilm assays
- Post Market product testing – Comparison assessment/ Competitive advantage
- Ex vivo porcine/ human skin models – wound healing claims
- Preclinical studies for different wound healing therapies