As a trusted CRO, NAMSA has assisted with the successful clinical research, development and commercialization of a wide range of orthopedic projects including arthroscopy, Cranio Maxillofacial Fixation (CMF), joint reconstruction, shoulder replacement spinal surgery, trauma fixation, orthobiologics, orthopedic bone cement materials, orthopedic power tools, consumables and beyond.
In addition to numerous biological safety studies, NAMSA has completed over 450 customized orthopedic preclinical studies worldwide.
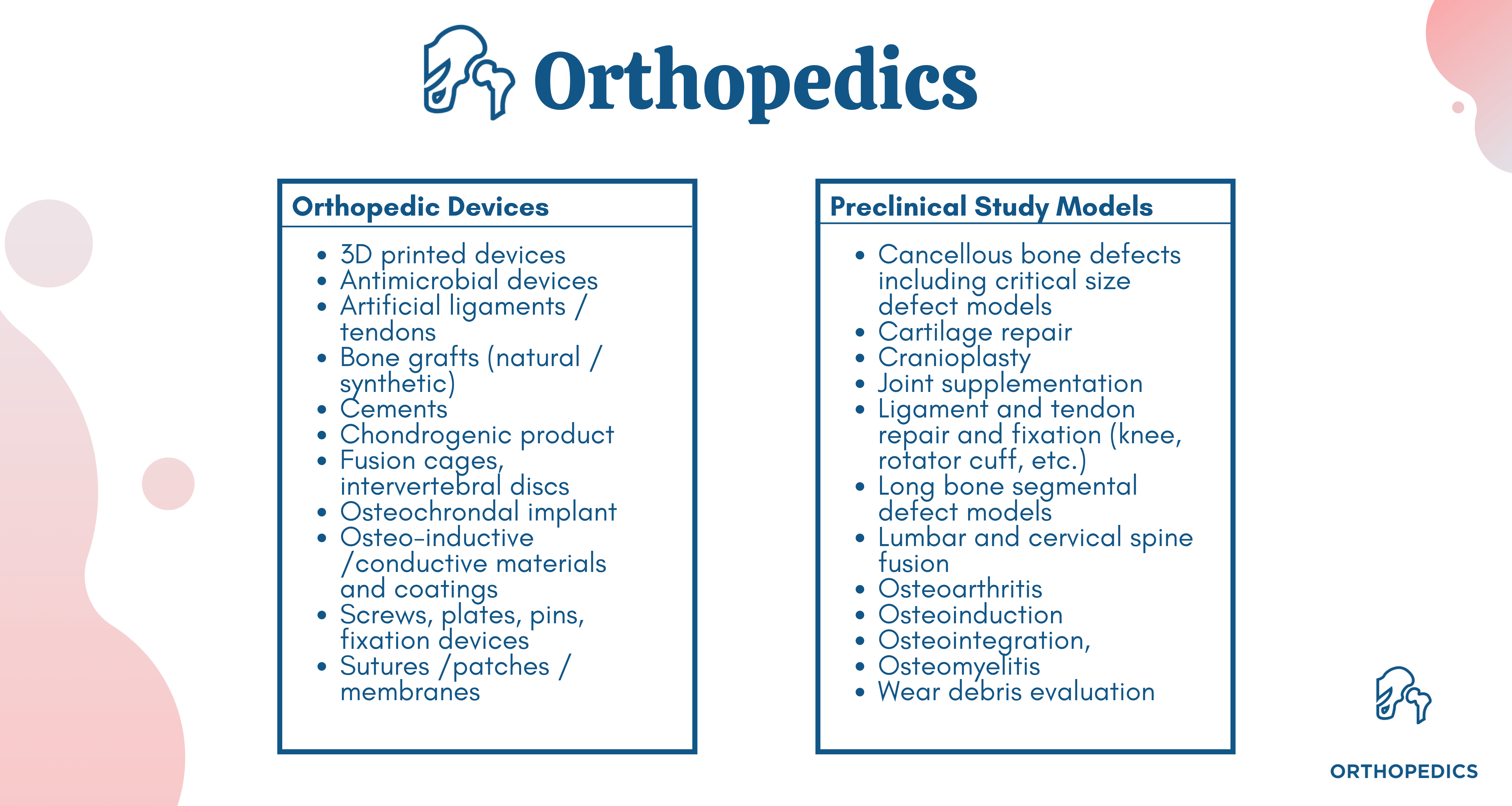
Device tests and models available at our global laboratories include:
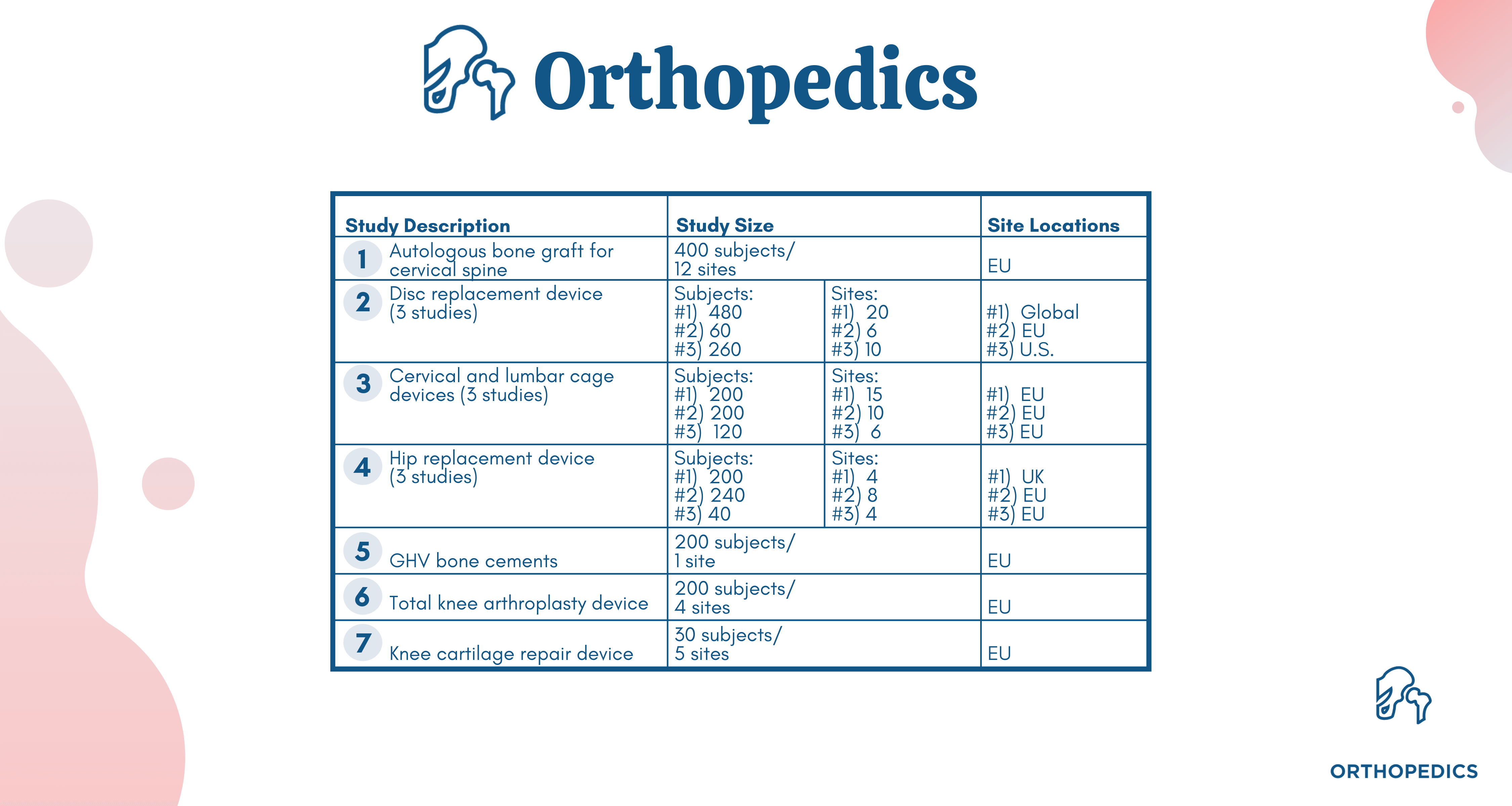
NAMSA is also well-versed in clinical research trials for orthopedic devices and understands the unique challenges faced by manufacturers and sponsors of these device types. Below is just a sampling of our wide-ranging experience with clinical studies around the globe: