The monkeypox (MPX) virus was first identified in 1970 with limited spread to East and West African nations. However, since January 2022 the monkeypox (MPX) virus has spread into regions absent of previously documented cases and is showing the first sustained chain of transmission without direct links to West or Central Africa. During this growing outbreak, the U.S. Secretary of Human and Health Services (HHS) determined a substantial need to open an Emergency Use Authorization (EUA) to combat the spread of the monkeypox disease in the United States. The FDA has responded with a policy to expand monkeypox vaccines and testing access. Similar to the COVID-19 EUA pathway, FDA templates are available for manufacturers interested in bringing a monkeypox (MPX) PCR IVD assay to market. Other templates for home testing kits, point-of-care rapid diagnostics, or over-the-counter testing are currently in process. At this time, other global regulatory bodies have not published abbreviated paths to market, and IVD applications should proceed via the normal approval pathways.
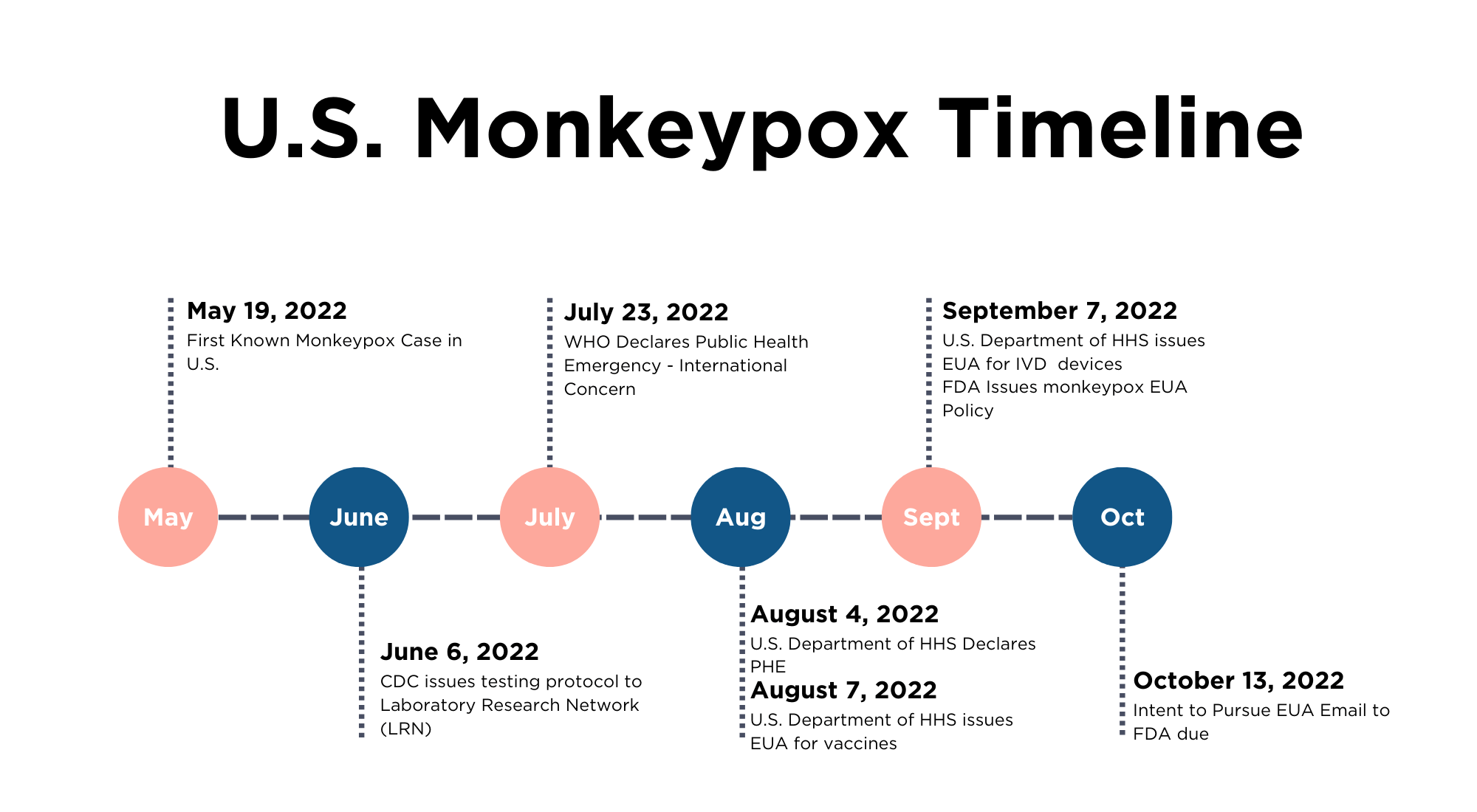
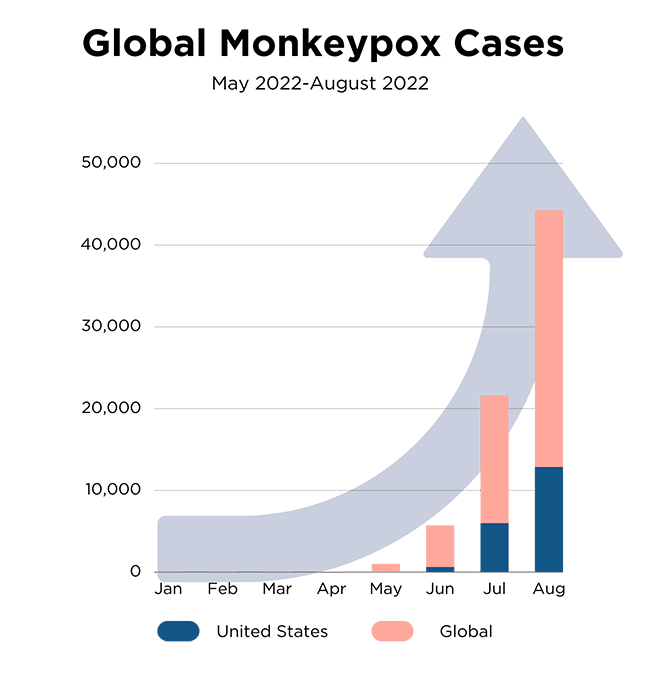
The U.S. identified the first monkeypox case on May 19, 2022. Since May, the cases have increased to more than 65,000 globally, with a third occurring in the United States. Currently, one FDA 510(k) cleared test is available for high-complexity laboratories (CDC Non-variola Orthopoxvirus Real-time PCR Primer and Probe Set (K181205, K221658, K221834 and K222558)). The CDC provided this test to public health agencies and select reference laboratories to increase access to testing. However, the increased case count has intensified the need for critical monkeypox test kits from experienced laboratories and developers.
For the above reasons, FDA issued “Policy for Monkeypox Tests To Address the Public Health Emergency Guidance for Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff” on September 7, 2022. This policy provides recommendations for manufacturer’s wishing to pursue the EUA route. FDA will prioritize assays with a high-throughput diagnostic test, tests with at-home specimen collection and/or rapid diagnostic tests from experienced developers. Additionally, the FDA will prioritize manufacturers with a high manufacturing capacity and shorter production ramp-up times.
As monkeypox cases continue to increase, partnerships with an experienced Contract Research Organization (CRO), such as NAMSA, can help to accelerate a manufacturer’s time to market via the EUA pathway. Our IVD team is experienced with the necessary FDA templates and can alleviate delays. Leverage our team’s experience to help move your device to the diagnostic market and support this public health initiative.
Please see our list of EUA services below:
EUA Strategy
Consulting on the manufacturer’s design and application to FDA Prioritization Criteria (High Throughput, home specimen collection, rapid test, experienced developer and manufacturing capacity).
EUA Intent to Submit
Preparation of Sponsor’s letter of intent to FDA. Consulting is available to help manufacturers provide the right information to the FDA in an acceptable format.
EUA Labeling
Review and provide edits on existing IFU, Box Labels, Vials Labels and other labeling to be compliant with EUA requirements.
Pre-EUA Submission
Compile and submit the pre-submission packet to the FDA to confirm the EUA clinical testing protocol is acceptable.
EUA Clinical Study
Collaborate with NAMSA on protocol writing, biostatistics support, data management or monitoring or NAMSA can fully manage the Sponsor’s prospective clinical trial.
EUA Submission
Compile all documentation provided by the Manufacturer into the EUA Template for Developers of Molecular Diagnostic Tests for monkeypox for FDA submission.
EUA Review & Gap Analysis
Review completed EUA Template for Developers of Molecular Diagnostic Tests for monkeypox for gaps and recommended remediation actions to be completed prior to submission to the FDA.
NAMSA IVD offers full support for your Global Regulatory, Clinical and Biostatistics/Data Management. Our full line of services can be found at namsa.com/IVD.
Complimentary Consultations
NAMSA, as the industry leader in IVD regulatory knowledge and product development, is proud to have collaborated with numerous U.S. and international IVD Sponsors for Emergency Use Authorization (EUA) assay submissions to combat COVID-19. If you are seeking expedited EUA development for monkeypox assays, or need assistance with other IVD regulatory, clinical, reimbursement, or preclinical studies, please reserve your complimentary consultation to learn more: namsa.com/subject-matter-experts/.

NAMSA’s IVD experts are committed to monitoring the U.S. and Global public health response to monkeypox. Please review the complimentary resources we have provided. As this situation evolves, we will continue to post key findings to help manufacturers navigate another public health emergency.
Receive free complimentary consultation from one of our EUA Specialists by completing the contact us form.
Resources
IVD Regulatory Guidance:
FDA Monkeypox Emergency Use Authorization for Medical Devices
WHO Monkeypox Outbreak 2022
IVD Clinical Guidance:
Blog Post: Monkeypox – Not All EUAs Are Created Equal by Wendy Schroeder
