Your cart is currently empty!
Deep Preclinical and Clinical Expertise in Numerous Categories
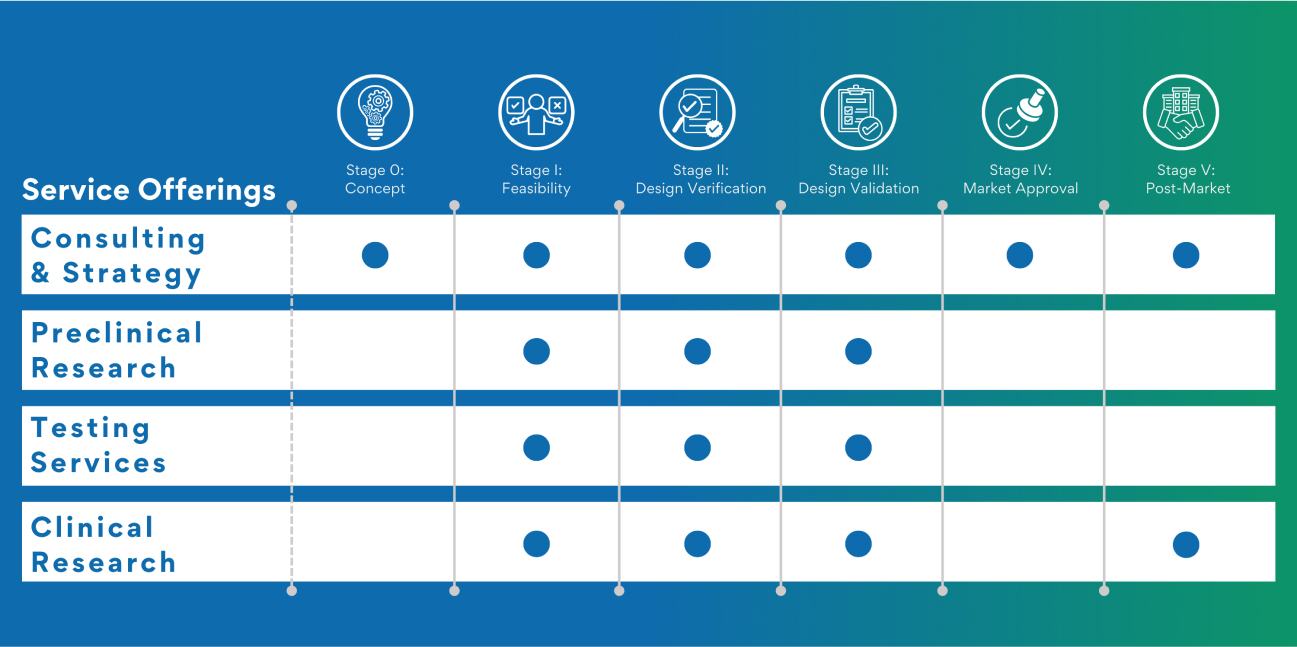
NAMSA has worked with tens of thousands of devices since 1967 but we have developed particular expertise in the device categories shown below.
All Services
Every year more than 3,000 medical devices and In Vitro Diagnostic companies worldwide trust NAMSA to help them bring innovative new products to market or ensure the ongoing safety of their existing products. Our team of 1,400 specialists are ready to help you conduct market research, preclinical studies, clinical trials, biological safety testing, regulatory submissions, and much more.

From startups to multinationals, NAMSA has been serving the medical device and IVD industries since 1967 and has a highly experienced team ready to serve you.

Assistance with biological evaluation plans and reports, FDA, EU MDR and IVDR submissions, market research, post-market compliance, and more.
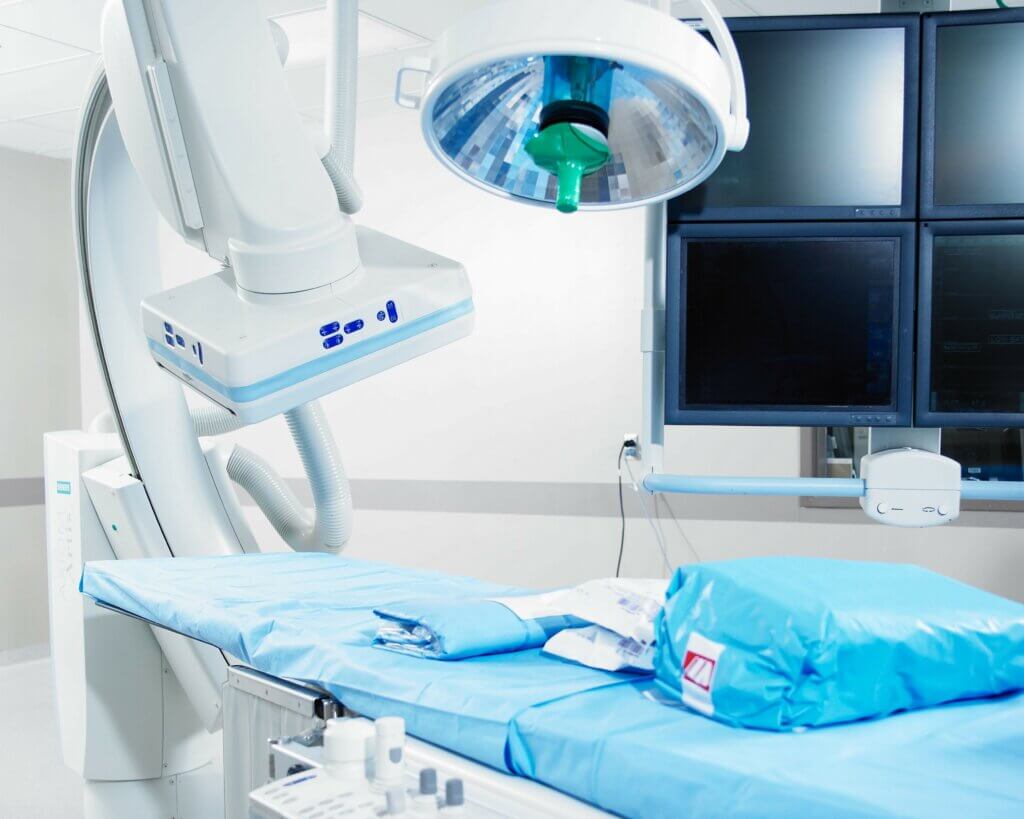
Unparalleled preclinical laboratory support for sponsors at GLP-compliant and AAALAC-accredited facilities in the United States and Europe.

Extensive medical device and IVD clinical trial expertise spanning all stages of clinical research. Global footprint provides clients direct access to local networks.
State-of-the-art labs conduct 118,000+ biocompatibility, microbiology, sterility and other tests annually. No other company conducts more tests.
NAMSA has built a reputation for excellence based on the deep industry expertise and client service offered by our team. Meet the consultants, scientists, medical writers, toxicologists, biostatisticians, surgeons and other experts who make NAMSA the preferred choice of device and IVD companies worldwide.






We work with a wide range of medical device and IVD manufacturers, from startups developing their first device to multinational companies with thousands of SKUs. No matter what your need is, our highly refined internal processes allow us to efficiently handle everything from routine medical device testing to long-term clinical trials.
Medical Device Clients Served Each Year
Medical Device Clinical Projects Last Year
Medical Device Tests Conducted Last Year
Global Lab Locations Doing Device Testing