Your cart is currently empty!
MDR Clinical Evaluation Plan and Report Writing (CEP and CER) for Medical Devices
Why Companies Choose NAMSA
Of Our Writers Have Advanced Degrees (MSc, PhD, MD)
Staff Consultants with EU Notified Body Experience
Clinical Evaluation Plans and Reports Prepared Annually
Whether your medical device has been on the EU market for decades, or you are pursuing a new CE Marking certification, NAMSA’s regulatory and medical writing teams are ready to help you gather, assess, and evaluate all necessary clinical evidence to support your submission or maintain postmarket compliance.
Quick facts about clinical evaluation:
- Clinical evaluation is mandated in Annex XIV of the EU Medical Device Regulation (2017/745)
- Your Clinical Evaluation Plan (CEP) provides a roadmap of how you will gather and evaluate safety and performance data
- There are 5 phases of clinical evaluation, which can be seen below
- For certain devices, clinical evidence can be based on devices that have equivalent technical, biological, and clinical characteristics
- Clinical evaluations must be performed throughout the entire life cycle of your medical device
Stages of Medical Device Clinical Evaluation
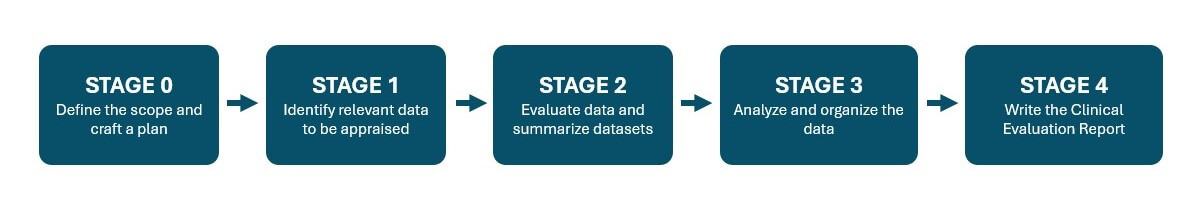
Create a Clinical Evaluation Plan (CEP) Your Notified Body Auditor Will Appreciate
As one of the largest medical device clinical research organizations, we know what Notified Bodies expect to see in clinical evidence submitted by manufacturers. NAMSA has helped thousands of device manufacturers gather the data to support regulatory submissions and maintain postmarket compliance. Among other things, your medical device CEP will clearly identify:
- Scope and objectives
- The intended purpose, indications for use, and clinical benefits of your medical device
- Methodology and study design
- How clinical data will be collected and analyzed
- How identified risks will be assessed
- How relevant published literature will be found and reviewed
- How postmarket data will be collected and evaluated
Our specialized team of medical writers and regulatory experts will work to define the data needed to support compliance with EU MDR Article 61, Annex XIV, XV or XVI, and the General Safety and Performance Requirements (GSPRs).
Gathering Clinical Evidence to Support Your MDR-Compliant Clinical Evaluation Report (CER)
Have you defined appropriate safety and performance endpoints? Are your clinical literature reviews adequate? After thoroughly reviewing your existing clinical evidence (if any exists), the NAMSA team will identify gaps in your data and build the clinical evaluation plan around those findings. The extent of data needed will depend on:
- Risk classification of your device and novelty of technology
- Claims you make about the device’s performance
- Existing device with CE vs. new device submission
- Whether your design/intended use/indications have changed since the last CER update
- How much real-world device use data you have
After establishing a plan, NAMSA will gather applicable supporting data from various sources, including:
- Systematic literature reviews
- Complaint and PMS data
- Preclinical, pivotal or postmarket studies
- Patient registries
Writing or Updating Your Medical Device CER
Built on the foundation of a solid clinical evaluation plan, our trained medical writers will organize, interpret, and present the data in your Clinical Evaluation Report (CER). Because we do so many medical device CERs each year, we can complete each project efficiently and to Notified Body expectations. NAMSA provides clinical evaluation plans and reports covering all device classes and therapeutic areas, including combination products. In addition, our team of former Notified Body executives support the strategy and development of CER, providing an extra measure of assurance that your CER will satisfy your auditor. Our goal is to make sure your upcoming certification or surveillance audit goes as smoothly as possible.
Request a Meeting with a NAMSA Clinical Evaluation Expert
Meet Our Team of MDR Regulatory Experts and Medical Writers
-
Áine Mary Duffy, BSc (Hons), PhD
 Clinical & Regulatory Operations ManagerView Bio
Clinical & Regulatory Operations ManagerView Bio -
Sean Bird, PhD
 Principal Medical WriterView Bio
Principal Medical WriterView Bio -
Caroline Guidicelli, MSc, MEng
 Principal Medical WriterView Bio
Principal Medical WriterView Bio -
Séverine Oudin-Fantin, PhD
 Manager–EMEA Medical Writing ServicesView Bio
Manager–EMEA Medical Writing ServicesView Bio -
Aurélie San Juan, MSc, PhD
 Principal Medical Writer, RegulatoryView Bio
Principal Medical Writer, RegulatoryView Bio
Related Services That May Interest You

EU MDR Technical Documentation

PMCF Plans, Surveys and Reports
