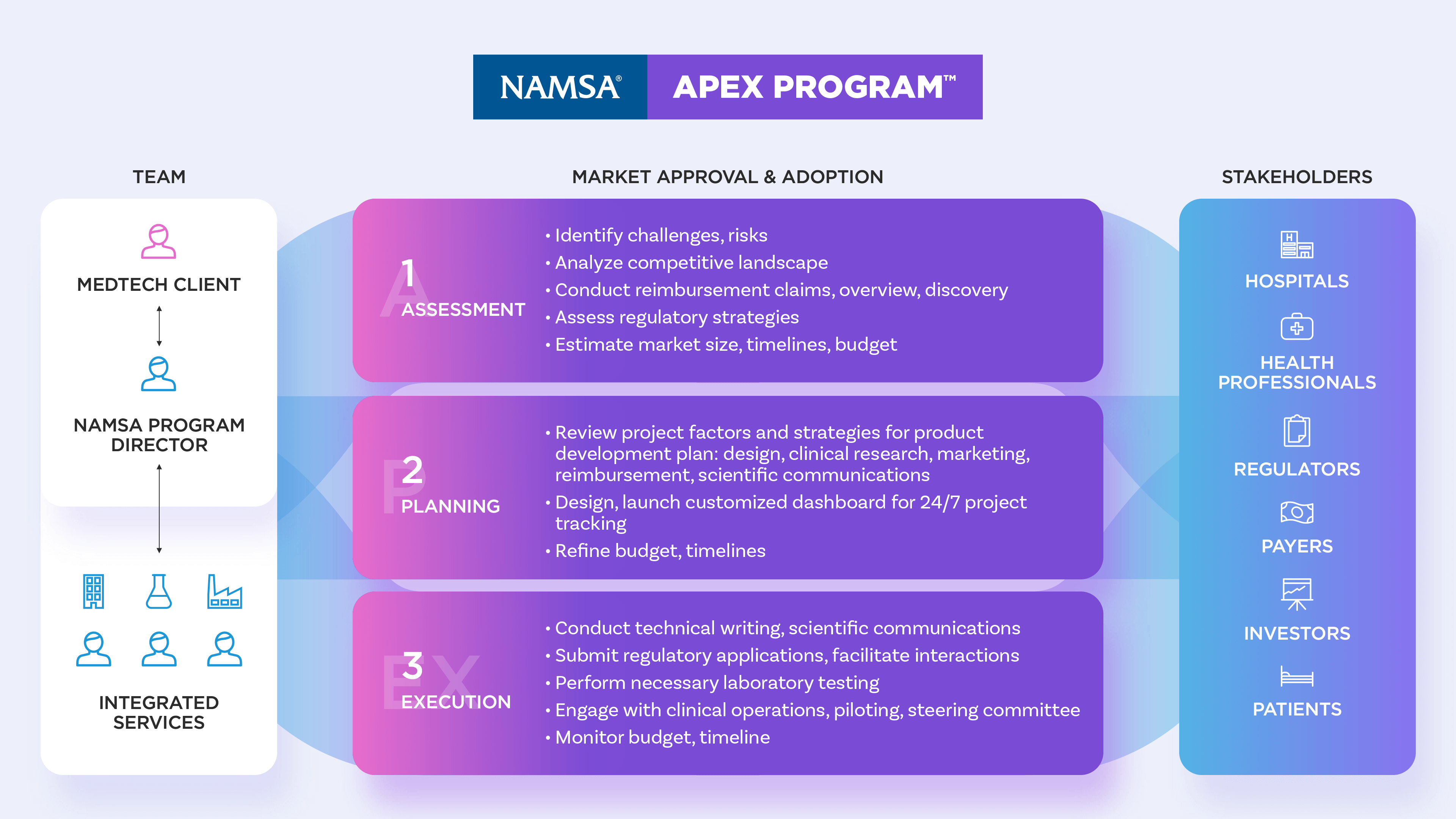
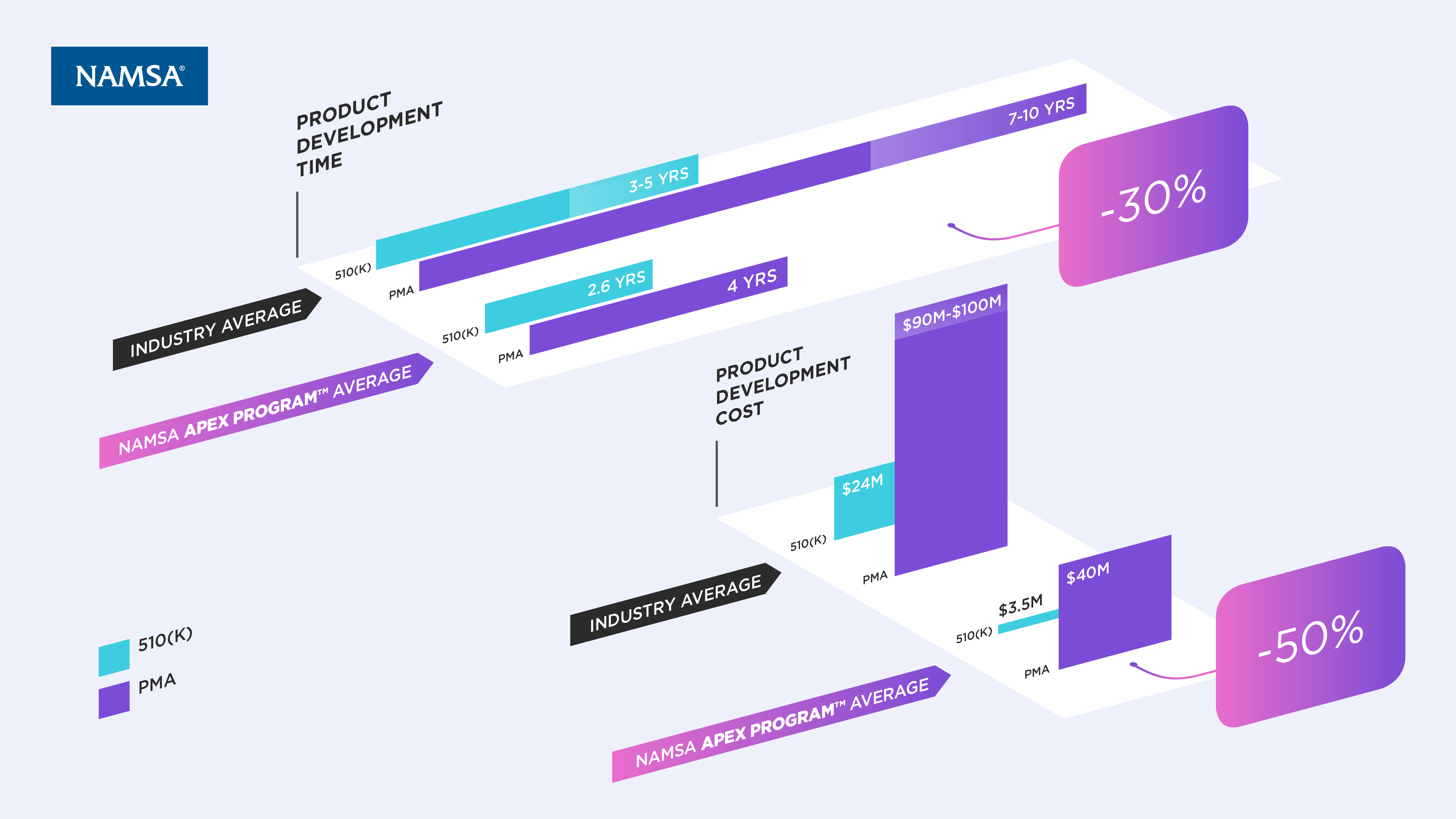
Medical device development is complex. In today’s value-based environment, the commercial challenges are more numerous than ever as regulatory approval does not guarantee market adoption. To mitigate risk, preserve capital and ensure the most efficient path to commercial success, development functions must be aligned with an integrated strategy.
The NAMSA APEX Program™ is designed with these objectives in mind. We help you succeed through our people—top Subject Matter Experts in the MedTech industry—and our processes—proven, integrated services and tools that deliver predictable planning, phase overlap and vertical integration of all product development phases. Our integrated consulting, lab and clinical approach ultimately delivers Sponsors significant time savings, cost reduction and accelerated commercialization.